About Us
Qualimax Consultants
Your Trusted Partner in Pharmaceutical Consulting & Compliance



We provide expert pharmaceutical consulting
and compliance solutions.
we are dedicated to providing comprehensive pharmaceutical consulting services that empower organizations to achieve excellence in quality, compliance, and operational performance. With a team of seasoned industry professionals
At Qualimax Consultants, we believe that strong quality systems form the foundation of every successful pharmaceutical organization. By integrating regulatory knowledge with hands-on industry experience, we help our clients strengthen compliance, streamline processes, and achieve consistent product quality across all operations.
Our commitment extends beyond compliance — we aim to build long-term partnerships based on trust, transparency, and continuous improvement. Qualimax delivers reliable, end-to-end consulting support at every stage of your quality journey.
Our Best Service
Expert Support Across Audits,
Validation, Training, and Quality Systems.

GMP Audit Services
GMP is a globally recognized set of regulations designed to ensure that products are manufactured in a controlled, consistent, and high-quality environment.

Internal Audit Services
We conduct detailed internal audits covering facilities, equipment, and daily operations—ensuring continuous GMP compliance, data integrity, and quality improvement across all departments.

Data Integrity Audits
We evaluate data governance, system controls, and documentation practices to ensure ALCOA+ compliance and reliable, traceable records across all GMP operations.

Gap analysis audits
We conduct in-depth gap analysis audits to identify discrepancies between current practices and GMP or regulatory requirements—helping you achieve full compliance and operational excellence.

Risk Assessments
Identifying, evaluating, and controlling process risks Implementing ICH Q9–based risk management tools

SOP Writing
We create clear, compliant, and user-friendly Standard Operating Procedures (SOPs) that align with cGMP and regulatory expectations. — ensuring every SOP reflects current practices, minimizes errors, and stands audit-ready.

Compliance Support
We ensure ongoing compliance through effective CAPA implementation, deviation control, and audit follow-ups—driving continual improvement and adherence to global GxP standards.

Technical Trainings
We deliver hands-on technical training programs to enhance employee competency in GMP practices, equipment handling, documentation, and regulatory compliance.

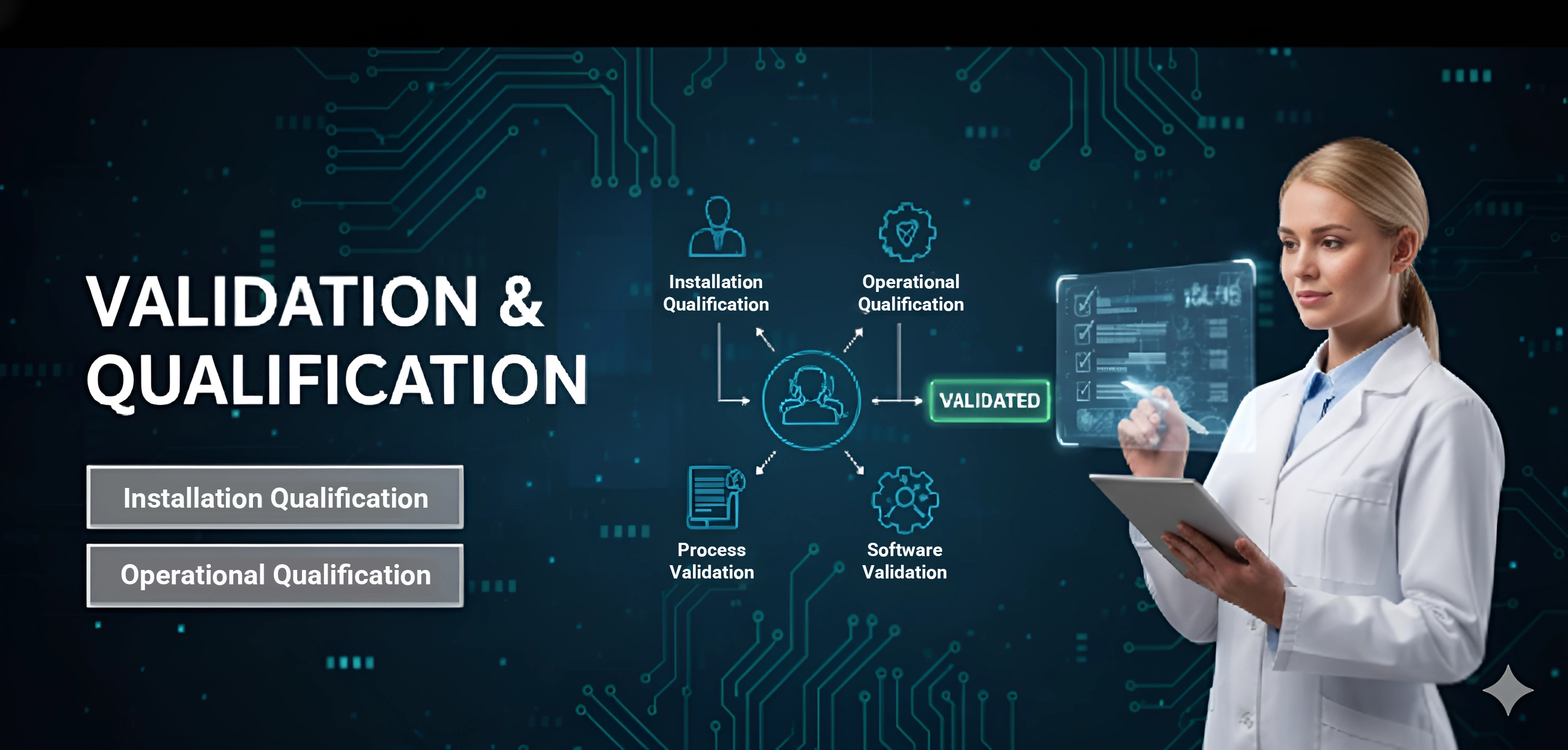
Validation & Qualification
We ensure systems and processes are qualified and validated through protocol design, execution, and review to meet regulatory standards.

Computer System Validation
We validate computerized systems to ensure data integrity, compliance with GxP guidelines, and consistent, reliable performance across all operations.
Why Choose Us?
At Qualimax Consultants, we combine deep regulatory knowledge with practical industry experience. Our expert team ensures your organization stays compliant, audit-ready, and quality focused.
Regulatory & Industry Expertise
Our team brings in-depth knowledge of global pharmaceutical regulations, ensuring strong support in audits, QMS implementation, and validation activities.
customized & Practical Solutions
We customize our services to fit your operational needs—covering audits, QMS support, training, and qualification activities.
Audit & Compliance Excellence
Stay inspection-ready with our expert guidance. We help you minimize compliance risks and build a strong quality culture.
Efficient Project Execution
We drive seamless project planning and execution — from QMS implementation to qualification and training — ensuring clear milestones, proactive coordination, and on-time, compliant delivery.
Cost-Effective Quality Solutions
We deliver high-impact quality and compliance solutions that optimize resources, reduce risks, and maximize long-term value.
Qualification & Validation Mastery
Our experts execute IQ, OQ, PQ, cleaning validation, process validation, and qualification with complete documentation and regulatory alignment.
Our Blogs
Frequently Asked Questions
Have questions about our pharma consulting and compliance services? We’re here to help.
We focus on building a strong quality culture, improving operational efficiency, and ensuring your systems and processes align with GMP, GLP, GDP, and WHO guidelines.
 ?
?
What are Pharma Consulting Services?
The pharmaceutical industry is a high-stakes environment where quality is a matter of life and death. Ensuring the quality of pharmaceutical products is essential to protecting public health and safety.
What is GxP and why is it important?
GxP stands for Good Practices, including GMP (Manufacturing), GLP (Laboratory), GDP (Distribution), and GCP (Clinical). Compliance ensures product quality, patient safety, and regulatory adherence.
Why should my company hire a Pharma Consultant?
- Ensure regulatory compliance
- Maintain product quality and safety
- Reduce operational and compliance risks
- Prepare for audits and inspections
- Implement industry best practices efficiently
Are your services customized for different organizations?
Yes. Qualimax Consultants provides customized solutions based on your company’s size, processes, and regulatory requirements.
Do you provide support for regulatory inspections?
Absolutely. We guide your team through regulatory inspections, provide mock audits, and ensure your documentation, systems, and processes are fully compliant.
Contact Us
We’d love to hear from you. Fill out the form and we’ll get back to you shortly.