Role of a pharma consulting Service in qualification and validation
Our validation strategies are customized to your processes and regulatory landscape. We incorporate both traditional and continuous process validation. We establish robust protocols that define critical parameters and acceptance criteria with real-time analytics throughout the lifecycle of your product.

Excellence in Qualification and Validation
When quality is built-in, confidence follows. Our qualification and validation services ensure every system and process performs exactly as intended — safe, compliant, and audit-ready.
We deliver a structured approach encompassing User Requirement Specification (URS), Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). From cleanrooms to critical utilities, our experts verify design integrity, functionality, and performance in line with international GMP standards — ensuring lasting compliance and reliability.
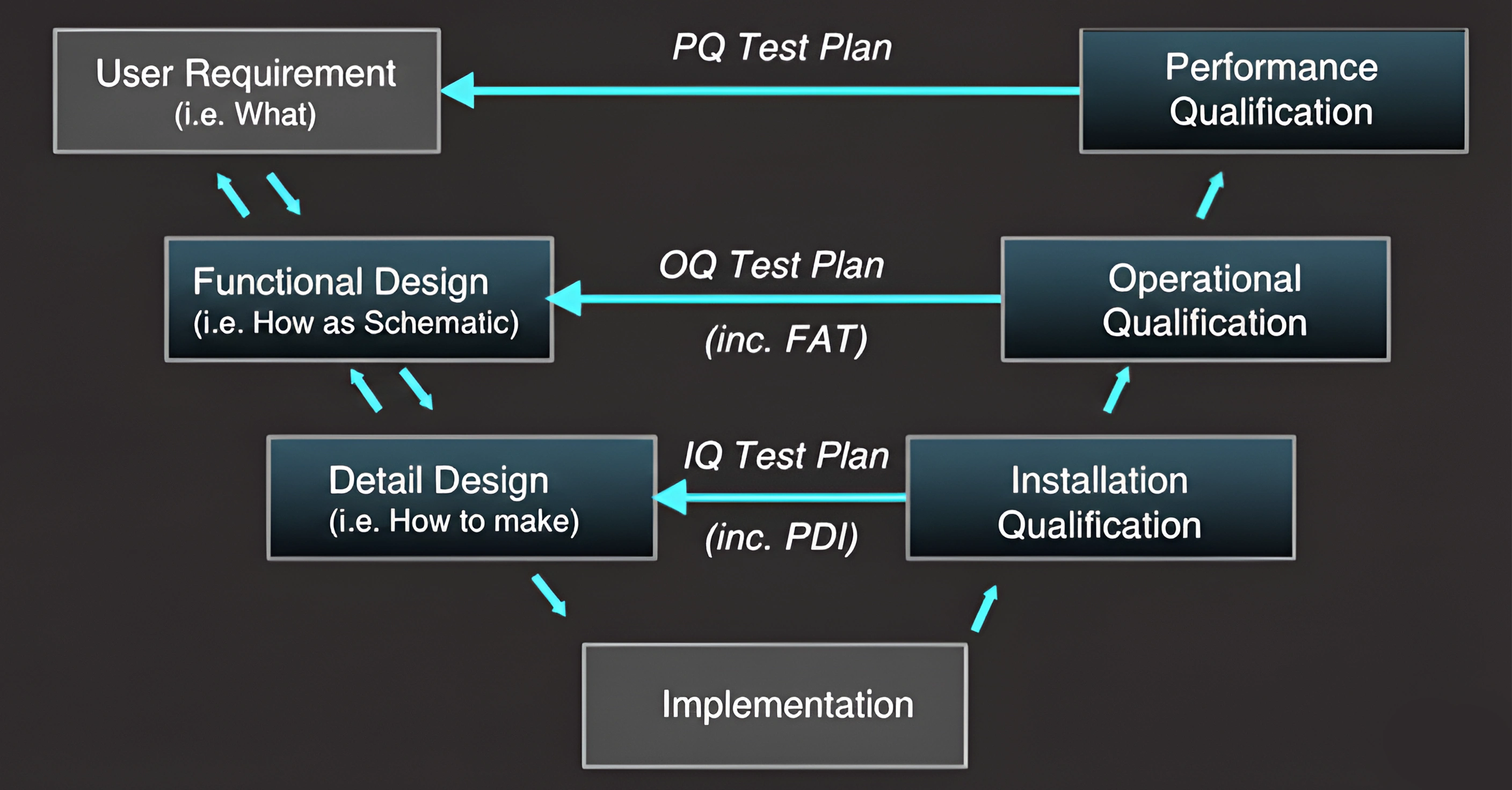
How Our Experts Qualify Systems
1. User Requirement Specification (URS) :
We define what the user expects from the system — performance, functionality, and compliance. These form the foundation for design and qualification.
2. Design & Functional Specifications (DS / FS) :
Our experts convert requirements into technical and functional specifications, describing how the system will meet operational goals.
3. Installation Qualification (IQ) :
We verify that all components, utilities, and documents are installed as per approved design and ready for operation.
4. Operational Qualification (OQ) :
Testing confirms the system functions correctly, with all controls, parameters, and safety features operating as intended.
5. Performance Qualification (PQ) :
Finally, we prove the system performs reliably in actual working conditions — meeting all URS expectations and ensuring readiness for GMP use.

How Our Experts Validate Procedures
1. Process Understanding :
We begin by studying the process flow, critical parameters, and associated risks to define validation scope and objectives.
2. Protocol Development :
Our experts prepare detailed validation protocols outlining acceptance criteria, sampling plans, test methods, and documentation requirements.
3. Execution & Data Collection :
Validation runs are performed under controlled conditions, with real-time monitoring to capture performance and variability data.
4. Data Review & Analysis :
Collected data is statistically analyzed to verify reproducibility, consistency, and compliance with acceptance limits.
5. Reporting & Approval :
A comprehensive validation report summarizes results, deviations, and conclusions, ensuring the procedure is approved for routine GMP use.

End-to-End Support for a Seamless Project
1. Project Planning & Coordination :
We define clear project requirements, timelines, and responsibilities to ensure seamless collaboration between your teams and ours.
2. Documentation Excellence :
We prepare all required documentation — including Validation Master Plans, IQ/OQ/PQ protocols, and summary reports — ensuring each document is thorough, accurate, and audit-ready.
3. Execution & Oversight :
Our experts supervise protocol execution and involved in the compliance of deviations .
4. Review & Handover :
We provide comprehensive reviews, approvals, and traceability documentation for a fully compliant and seamlessly executed project.
